

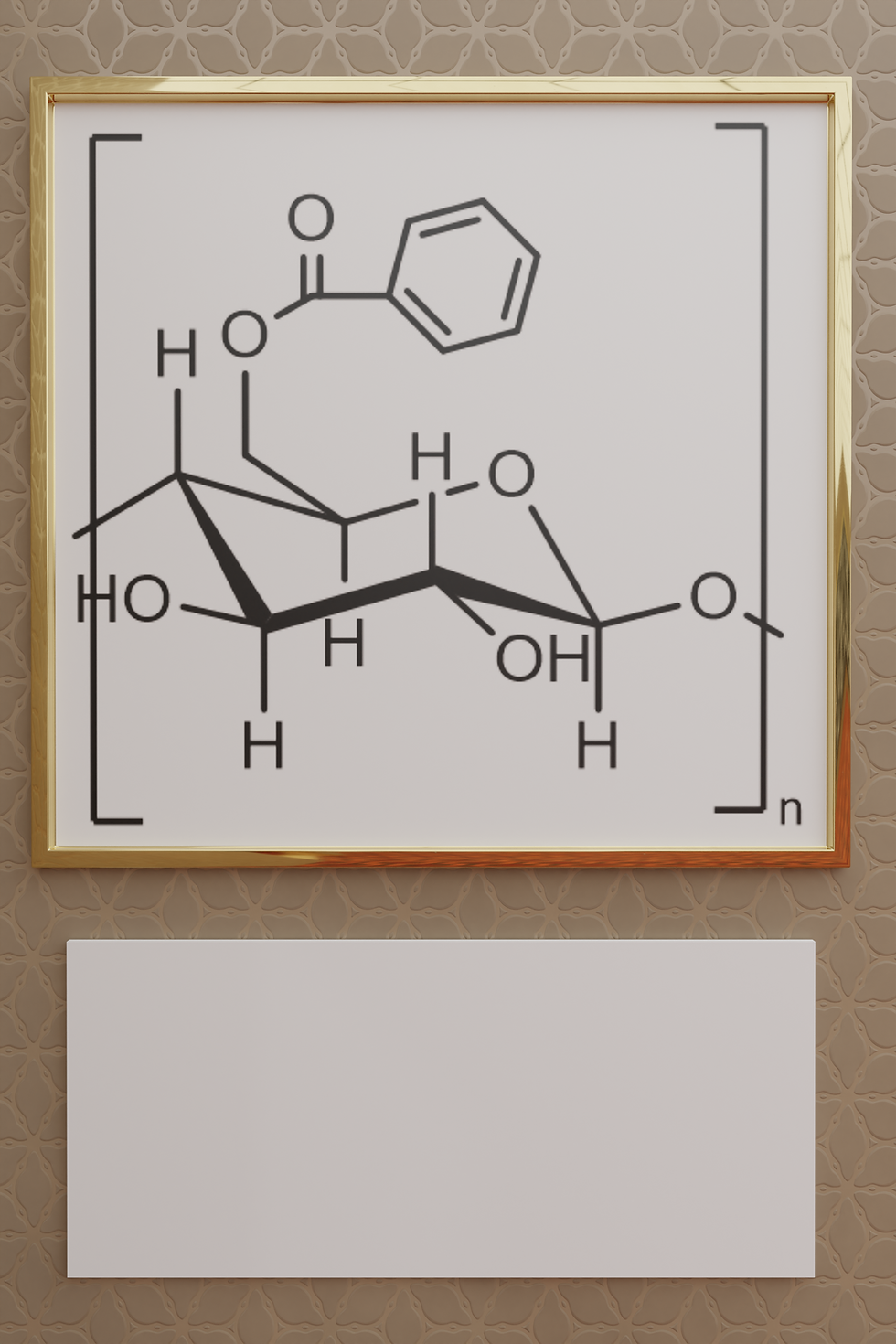
Schematic representation of benzylated starch via an esterification reaction
Schematic representation of benzylated starch via an esterification reaction
Modifying Starch
Starch is one of the best candidates to replace non-renewable plastics thanks to its wide availability and low cost and it is already widely used in textile, food, biomedical, packaging and paper industries. However, some of the physicochemical characteristics of native starch such as high brittleness, low thermal stability and hydrophobicity are undesirable for many applications. Chemical modification of starch is necessary to fine-tune its properties for the intended final use and unlocking the full potential of this sustainable bio-material.



Starch
Above you see a diagrammatic representation of the lamellar structure of a starch granule. (A) Stacks of microcrystalline lamellae separated by amorphous growth rings. (B) Magnified view of the amorphous and crystalline regions. (C) Double helical structures (reproduced from Tester et al.[1])
Starch is the most abundant natural polysaccharide after cellulose, it is produced by plants as an energy storage molecule and it is found in roots, seeds and leaves. Starch is composed of two macromolecules: amylose, a linear polymer of glucose units linked by α-(1→4) linkages, and amylopectin, a highly branched polymer of glucose units linked by α-(1→6) linkages arranged in shorter chains. The ratio between amylose and amylopectin changes depending on the botanical origin of the starches with amylose usually accounting for 70-80% in weight of starch composition. Amylopectin and amylose are arranged in granules made of alternating amorphous and semi-crystalline growth rings. The crystalline lamellae are composed mainly of amylopectin while the amorphous regions are occupied mainly by amylose chains. Starch granule size varies in size from 1 to 100µm, depending on the botanical source.
Modifying
Starch can be modified to tailor its properties for the final use via physical or chemical modification techniques. Physical modifications do not alter the chemical structure of the starch molecule and include heat-moisture, ultrasonic, high and ultra-high pressure, pulsed electric field, microwave irradiation and milling treatments. While having the advantages of low-cost and absence of any extra chemical agents used, physical modifications only allow for minor changes of the treated starches and such modifications are usually not as thermostable as the ones achieved via chemical modification.
The presence of 3 reactive OH groups on the positions 2, 3 and 6 of the anhydroglucose unit (AGU) of starch allow for an wide array of chemical reactions to be performed [2,3]. Some of the most common starch chemical modifications are: cationization, oxidation, etherification, esterification, cross-linking and grafting. One very interesting modification is the introduction of benzylic groups along the the starch backbone, typically via esterification or etherification reactions. The resulting benzylated starch has increased hydrophobicity and could find applications in the paper coating industry as replacement for petroleum derived additives [4].
Initial results are promising, with clear signs of successful functionalization detected with spectroscopic techniques such as nuclear magnetic resonance (NMR) and Fourier-Transform Infrared (FTIR) spectroscopy and scanning electron microscopy (SEM).
Below you see SEM pictures of native (left) and benzylated (right) starch.


Supercritical CO2 as solvent
Starch is poorly soluble in water and most organic solvents due to the extensive network of intra- and inter-molecular hydrogen bonds between hydroxyl groups on the AGU units and the backbone of the chains. In aqueous systems the chemical reaction proceeds in an heterogeneous way, this reduces the reaction efficiency (RE) and makes it necessary to increase reaction times and/or reagents amounts to achieve the required functionalization. When performed in aqueous slurries, the distribution of the substituents in the modified starch is non-homogenous across the granule section, with most of the new groups being introduced on the surface of the granule and much fewer in the bulk.
The use of organic solvents such as DMSO and pyridine can help in achieving higher DS values thanks to better dissolution and catalytic action but the high toxicity and flammability of these solvents are not compatible with the current shift towards more environmentally friendly industrial production, hence there is great interest in novel, sustainable solvents to chemically modify starch in a “greener” way while still achieving the desired material properties.

Supercritical carbon dioxide (scCO2) has great potential as a green media for the modification of natural polysaccharides thanks to its relatively mild critical conditions (304K and 73.8MPa), low cost, low toxicity and inertness towards many chemical species. ScCO2 is easily removed from the reaction vessel by a simple depressurization step and it can be recycled in an environmentally-friendly way. One of the most interesting properties of scCO2 is the tunability of the solvent properties such as its density by a slight change in the supercritical parameters of T and P, allowing for the tailoring of the solvent to a specific reaction system [5–7]. The use of scCO2 in the modification of natural polymers is of particular interest as it would further reinforce the green credentials of such macromolecules. Indeed, much research has been performed in the past 20 years on the application of scCO2 to the modification of natural polysaccharides, but most of the published studies are focused on the physical processes such as the fabrication of aerogels or foams and their impregnation or drying, supercritical fluid extrusion and extraction of active components.
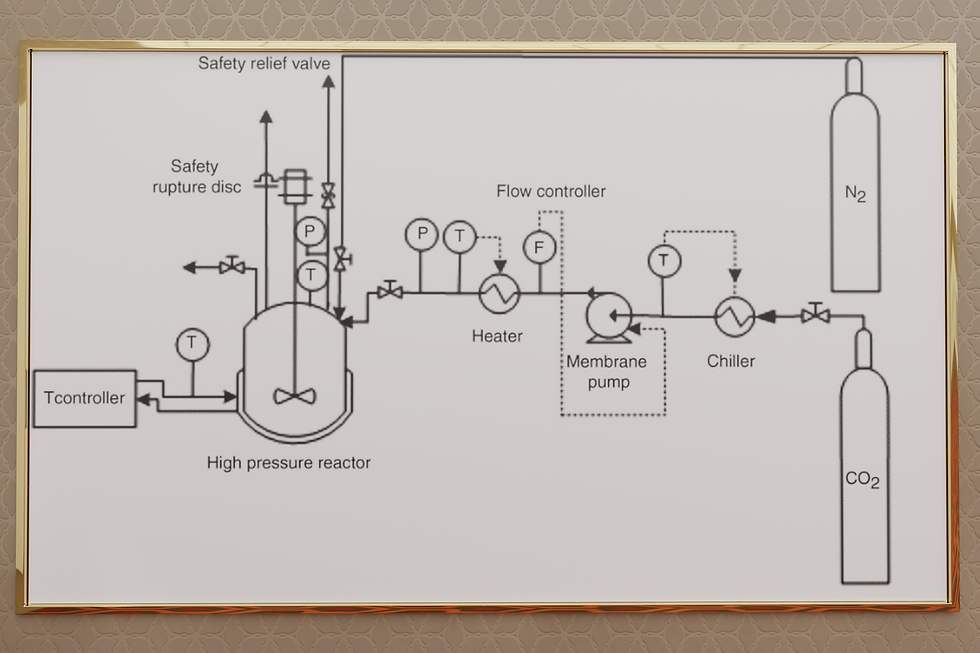
The use of supercritical CO2 as reaction media for the chemical modification of starch has been poorly investigated even though there are many advantages in the use of this fluid such as improved mass transfer thanks to the swelling of the starch granule promoted by the plasticizing effect of scCO2 that increases the mass transfer and hence the overall reaction efficiency. Moreover, the use of scCO2 is expected lead to a more even distribution of the chemical substituents along the granule section with resulting improved material properties. The aim of this present work is to investigate the benzylation of starch using scCO2 as reaction media testing a wide range of reaction conditions using the setup depicted below.


References
[1] R. F. Tester, J. Karkalas, and X. Qi, “Starch - Composition, fine structure and architecture,” J. Cereal Sci., vol. 39, no. 2, pp. 151–165, 2004, doi: 10.1016/j.jcs.2003.12.001.
[2] Y. F. Chen, L. Kaur, and J. Singh, Chemical Modification of Starch. Elsevier Ltd, 2018.
[3] N. Masina et al., “A review of the chemical modification techniques of starch,” Carbohydr. Polym., vol. 157, pp. 1226–1236, 2017, doi: 10.1016/j.carbpol.2016.09.094.
[4] J. Willem and J. Miel, Synthesis and application of hydrophobized waxy potato starch. 2014.
[5] F. Picchioni, “Supercritical carbon dioxide and polymers: An interplay of science and technology,” Polym. Int., vol. 63, no. 8, pp. 1394–1399, 2014, doi: 10.1002/pi.4722.
[6] Y. Fan and F. Picchioni, “Modification of starch: A review on the application of ‘green’ solvents and controlled functionalization,” Carbohydr. Polym., vol. 241, no. April, 2020, doi: 10.1016/j.carbpol.2020.116350.
[7] C. Boyère, C. Jérôme, and A. Debuigne, “Input of supercritical carbon dioxide to polymer synthesis: An overview,” Eur. Polym. J., vol. 61, pp. 45–63, 2014, doi: 10.1016/j.eurpolymj.2014.07.019.
[8] H. Muljana, F. Picchioni, H. J. Heeres, and L. P. B. M. Janssen, “Process-product studies on starch acetylation reactions in pressurised carbon dioxide,” Starch/Staerke, vol. 62, no. 11, pp. 566–576, 2010, doi: 10.1002/star.201000021.


